- Home
- Technology
- News
FDA approves human trials for Musk's neuralink brain implant
The Neuralink brain implant developed by Elon Musk has been granted FDA approval to proceed with human trials.


California: Elon Musk's Neuralink brain implant has received FDA approval for human trials, marking a significant milestone in the development of this groundbreaking technology.
The coin-sized device, which is implanted in the skull using ultra-thin wires connected directly to the brain, aims to revolutionize medical interventions and potentially transcend the limits of human abilities.
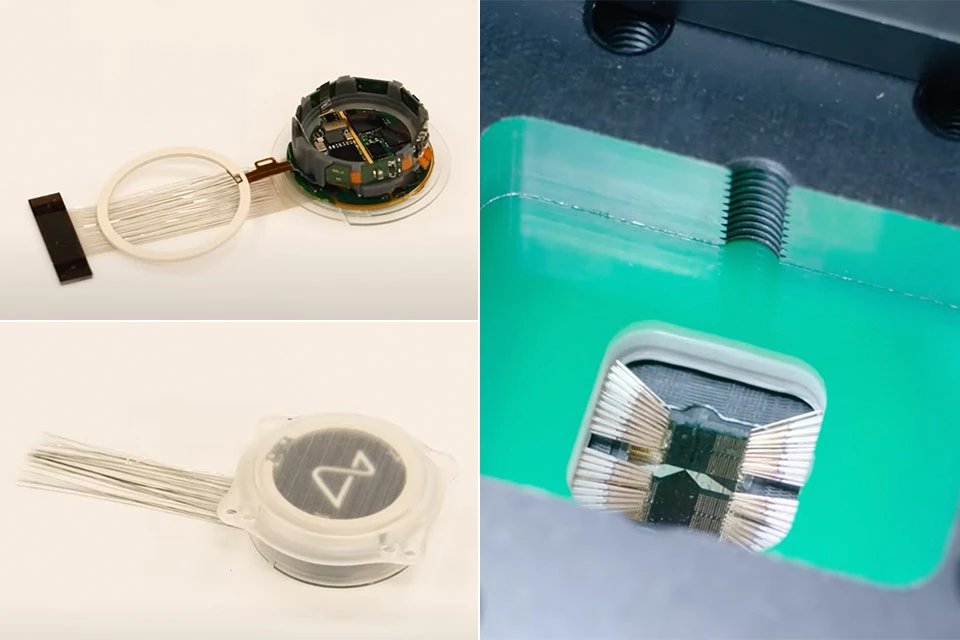
The initial focus of the human trials will be on restoring vision and assisting individuals with limited muscle control in operating digital devices.
However, the potential applications of the Neuralink implant go beyond medical purposes.
Elon Musk envisions a future where the technology can create a new kind of hybrid beings—part-human, part-robot cyborgs—capable of surpassing the capabilities of able-bodied humans.
Remarkable results have already been demonstrated during testing.
A monkey with the Neuralink implant achieved impressive control, reaching 65% and 88% of the median Neuralinker's precision cursor control using a mouse.
This progress fuels the belief that full-body functionality can be restored to individuals with severed spinal cords, pushing the boundaries of what was once deemed impossible.
Silver: NBA to explore relief for Heat over Rozier
- 5 hours ago
Is eliminated Chiefs' Super Bowl window closing -- and what changes are ahead?
- 5 hours ago
Heat F Jovic's MRI clean after hard fall; day-to-day
- 5 hours ago
Water aggression against Pakistan: India curtails Jhelum flows after Chenab
- 13 hours ago

Sony’s XM5 over-ear headphones are cheaper than ever — and they come with free wireless earbuds
- 6 hours ago
More than 42mn children administered polio vaccination during final polio drive in Pakistan
- 18 hours ago

Trump’s attack on trans health care, briefly explained
- 4 hours ago

Arcade1Up isn’t dead, maybe
- 6 hours ago

Climate change is rewriting polar bear DNA
- 4 hours ago

9 actually good things that happened to animals this year
- 4 hours ago
Harrison Ford to get lifetime acting award
- 13 hours ago

One of Trump’s grudges now threatens America’s weather forecasts
- 4 hours ago










